Beyond Weight Loss a Review of the Therapeutic Uses of Verylow Carbohydrate Ketogenic Diets
Abstruse
Very-low-sugar diets or ketogenic diets accept been in use since the 1920s every bit a therapy for epilepsy and tin can, in some cases, completely remove the need for medication. From the 1960s onwards they take go widely known as one of the most common methods for obesity treatment. Recent work over the last decade or so has provided show of the therapeutic potential of ketogenic diets in many pathological atmospheric condition, such every bit diabetes, polycystic ovary syndrome, acne, neurological diseases, cancer and the amelioration of respiratory and cardiovascular affliction risk factors. The possibility that modifying nutrient intake can be useful for reducing or eliminating pharmaceutical methods of handling, which are often lifelong with significant side effects, calls for serious investigation. This review revisits the meaning of physiological ketosis in the calorie-free of this testify and considers possible mechanisms for the therapeutic deportment of the ketogenic diet on different diseases. The present review besides questions whether there are even so some preconceived ideas almost ketogenic diets, which may exist presenting unnecessary barriers to their use every bit therapeutic tools in the doctor'due south manus.
Introduction
During contempo years, an increasing amount of evidence has accumulated in the literature, suggesting that very-low-carbohydrate ketogenic diets (VLCKD) could have a therapeutic role in numerous diseases. The use of VLCKD in treating epilepsy has been well established for many decades and these diets have get even more widely known, as they became popular in the 1970s for weight loss—especially equally the 'Atkins Diet'.one More recently, the therapeutic use of ketogenic diets in other diseases has been studied with positive results—it is an important direction for research considering, clearly, if nutritional intervention can reduce reliance on pharmaceutical treatments information technology would bring pregnant benefits from an economic as well equally a social point of view given the current US $750 billion almanac toll of pharmaceuticals.2
Ketogenic diets are characterized past a reduction in carbohydrates (unremarkably to less than 50 thou/solar day) and a relative increase in the proportions of protein and fatty.3 The noesis regarding the metabolic effects of classic ketogenic diets originates from the pioneering work of Cahill and colleagues in the 1960s,four but the realization of the importance of these diets from a clinical point of view can be traced back to the early 1920s when they began to exist successfully used in the handling of epilepsy.five There even appears to be a reference to its use in the Bible in the story of the cured epileptic (New Testament, Matthew 17:14–21). Alongside the huge amount of data about the influence of right nutrition on health status and affliction prevention (encapsulated in various nutritional guidelines delivered by public health committees worldwide), there is also ample bear witness to support the notion that a low-carbohydrate diet tin lead to an improvement in some metabolic pathways and have beneficial health effects. To utilise 'nutrient as medicine' is as attractive a concept equally it is aboriginal, and in the hope of realizing this much effort has been dedicated to exploring the furnishings of VLCKD on human metabolism. In this review we will look at all the areas where ketogenic diets have been proposed equally having potential clinical utility with a cursory discussion of the testify.
What is ketosis?
Insulin activates central enzymes in pathways, which store free energy derived from carbohydrates, and when at that place is an absence or scarcity of dietary carbohydrates the resulting reduced insulin level leads to a reduction in lipogenesis and fat accumulation. After a few days of fasting, or of drastically reduced carbohydrate consumption (below 50 thou/day), glucose reserves become insufficient both for normal fat oxidation via the supply of oxaloacetate in the Krebs cycle (which gave origin to the phrase 'fat burns in the flame of carbohydrate') and for the supply of glucose to the central nervous system (CNS).four
The CNS cannot apply fat every bit an energy source; hence, it unremarkably utilizes glucose. After 3–4 days without sugar consumption the CNS is 'forced' to notice culling energy sources, and as demonstrated by the archetype experiments of Cahill and colleaguesfour this culling free energy source is derived from the overproduction of acetyl coenzyme A (CoA). This condition seen in prolonged fasting, blazon 1 diabetes and loftier-fat/low-carbohydrate diets leads to the production of higher-than-normal levels of so-chosen ketone bodies (KBs), that is, acetoacetate, β-hydroxybutyric acid and acetone—a process chosen ketogenesis and which occurs principally in the mitochondrial matrix in the liver.6
The master KB produced in the liver is acetoacetate but the primary circulating ketone is β-hydroxybutyrate although the latter is not, strictly speaking, a KB because the ketone moiety has been reduced to a hydroxyl group. Under normal weather of adequate dietary carbohydrate, the production of free acetoacetic acrid is negligible and information technology is chop-chop metabolized past various tissues, especially the skeletal and heart muscles. In conditions of overproduction of acetoacetic acid, information technology accumulates above normal levels and function of information technology is converted to the other two KBs leading to ketonemia and ketonuria (presence of KBs in the blood and urine). The characteristic 'sweetness' jiff odor of ketosis is caused by acetone, which, existence a very volatile compound, is eliminated mainly via respiration in the lungs. The pathway that results in the formation of 3-hydroxy-iii-methylglutaryl–CoA from acetyl CoA also occurs in the cytosol of hepatic cells where it is used instead for the biosynthesis of cholesterol. Under normal conditions, the concentration of KBs is very low (<0.3 mmol/l) compared with glucose (∼4 mmol), and as glucose and KBs have a similar kM for glucose ship to the brain the KBs brainstorm to exist utilized as an energy source by the CNS when they reach a concentration of about iv mmol/50, which is close to the Km for the monocarboxylate transporter.3, half-dozen
KBs are so used by tissues every bit a source of energy3 through a pathway that leads to formation from β-hydroxybutyrate of 2 molecules of acetyl CoA, which are used finally in the Krebs cycle. It is interesting to note that the KBs are able to produce more energy compared with glucose because of the metabolic effects of ketosis—the high chemical potential of 3-β-hydroxybutyrate leads to an increment in the ΔM 0 of ATP hydrolysis.3 A further point to underline is, as shown in Table ane, that glycaemia, even though reduced, remains within physiological levels because of the fact that glucose is formed from 2 sources: from glucogenic amino acids and from glycerol liberated via lysis from triglycerides.seven
We would like to emphasize that ketosis is a completely physiological mechanism and it was the biochemist Hans Krebs who showtime referred to physiological ketosis to differentiate it from the pathological keto acidosis seen in type one diabetes.viii In physiological ketosis (which occurs during very-low-calorie ketogenic diets), ketonemia reaches maximum levels of 7/8 mmol/l (it does not get higher precisely because the CNS efficiently uses these molecules for energy in identify of glucose) and with no change in pH, whereas in uncontrolled diabetic ketoacidosis information technology tin can exceed 20 mmol/l with a concomitant lowering of blood pHnine, 10 (Tabular array ane).
Therapeutic roles of ketogenic diets
Strong evidence
Weight loss
There is no doubtfulness that at that place is potent supportive prove that the employ of ketogenic diets in weight-loss therapy is effective; yet, there are contrasting theories regarding the mechanisms through which they piece of work. Some researchers advise that there are non in fact whatsoever metabolic advantages in low-sugar diets and that weight loss results only from reduced caloric intake, probably due to the increased satiety event of protein.12 Others instead promote the hypothesis that there is indeed a distinct metabolic advantage, which has recently been explored in more than detail, raising interest in the function of VLCKD in weight loss and effects on metabolism in general.thirteen The first police of thermodynamics, also known as the law of conservation of energy, has in effect controlled the concepts for the basis of weight loss for over a century—resulting in a difficulty in accepting other means of thinking. Adhering to these traditional concepts the US Department of Agriculture has ended that diets, which reduce calories, will event in effective weight loss independent of the macronutrient composition, which is considered less important, even irrelevant.14 In dissimilarity with these views, the majority of ad-libitum studies demonstrate that subjects who follow a low-saccharide diet lose more weight during the offset three–half-dozen months compared with those who follow balanced diets.15, 16, 17 One hypothesis is that the use of energy from proteins in VLCKD is an 'expensive' process for the body and then tin can lead to a 'waste of calories', and therefore increased weight loss compared with other 'less-expensive' diets.thirteen, 18, 19 The average human torso requires 60–65 grand of glucose per twenty-four hours, and during the start phase of a diet very low in carbohydrates this is partially (16%) obtained from glycerol, with the major function derived via gluconeogenesis from proteins of either dietary or tissue origin.12 The energy toll of gluconeogenesis has been confirmed in several studies7 and it has been calculated at ∼400–600 Kcal/twenty-four hour period (due to both endogenous and food source proteins.xviii Despite this, in that location is no direct experimental show to support this intriguing hypothesis; on the opposite, a recent study reported that at that place were no changes in resting energy expenditure subsequently a VLCKD.20 A simpler, possibly more probable, explanation for improved weight loss is a possible appetite-suppressant action of ketosis. The mechanism for this is not established merely testify supports direct action of KBs together with modifications in levels of hormones, which influence ambition, such as ghrelin and leptin.21 Here we can summarize (listed in order of importance and available evidence) that the weight-loss effect of VLCKD seems to be acquired by several factors:
- 1
Reduction in appetite due to higher satiety effect of proteins,12, 22 effects on appetite command hormones21 and to a possible direct appetite-suppressant action of the KBs.23
- ii
Reduction in lipogenesis and increased lipolysis.7, ten
- 3
Reduction in the resting respiratory quotient and, therefore, greater metabolic efficiency in consuming fats.20, 24
- 4
Increased metabolic costs of gluconeogenesis and the thermic effect of proteins.thirteen, xviii
Cardiovascular disease
Several lines of evidence point to beneficial furnishings of VLCKD on cardiovascular hazard factors. In the past, in that location have been doubts expressed nigh their long-term safety and increased effectiveness compared with 'balanced' diets,25 and conspicuously negative opinions regarding possible deleterious effects on triglycerides and cholesterol levels in the blood.26 However, the majority of recent studies seem instead to amply demonstrate that the reduction of carbohydrates to levels that induce physiological ketosis (see above 'What is ketosis?' section) tin can actually lead to significant benefits in blood lipid profiles.xv, 17, 27 The VLCKD outcome seems to exist peculiarly marked on the level of blood triglycerides,24, 28 merely at that place are also pregnant positive effects on total cholesterol reduction and increases in high-density lipoprotein.24, 28, 29 Furthermore, VLCKD have been reported to increase the size and volume of depression-density lipoprotein–cholesterol particles,29 which is considered to reduce cardiovascular disease take a chance, as smaller low-density lipoprotein particles accept a higher atherogenicity. In that location are also directly nutrition-related effects on overall endogenous cholesterol synthesis. A key enzyme in cholesterol biosynthesis is 3-hydroxy-iii-methylglutaryl–CoA reductase (the target for statins), which is activated by insulin, which means that an increase in blood glucose and consequently of insulin levels will lead to increased endogenous cholesterol synthesis. A reduction in dietary carbohydrate will have the contrary effect and this, coupled with the additional inhibition by dietary cholesterol and fats on endogenous synthesis, is likely to exist the mechanism via which physiological ketosis can ameliorate lipid profiles. Hence, there are stiff doubts about the negative effects of dietary fats when they are consumed as part of a VLCKD, on cholesterol and triglycerides blood levels, whereas there are strong pointers to the beneficial effects of VLCKD on these cardiovascular gamble parameters.27, 28
Type two diabetes
Insulin resistance is the primary feature underlying type 2 diabetes (T2D) but it also exists across a continuum in the general population, and to varying extents it disrupts insulin activeness in cells, which tin can cause a wide spectrum of signs and symptoms. A primary feature of insulin resistance is an impaired power of muscle cells to take up circulating glucose. A person with insulin resistance will divert a greater proportion of dietary saccharide to the liver where much of it is converted to fat (that is, de novo lipogenesis), as opposed to being oxidized for energy in skeletal muscle.thirty Although Hellerstein31 has recently reported that de novo lipogenesis contributes only ∼20% of new triglycerides, this greater conversion of dietary carbohydrate into fatty, much of it entering the circulation as saturated fat, is a metabolic abnormality that significantly increases take a chance for diabetes and heart disease. Thus, insulin resistance functionally manifests itself as 'carbohydrate intolerance'. When dietary carbohydrate is restricted to a level beneath which it is not significantly converted to fatty (a threshold that varies from person to person), signs and symptoms of insulin resistance amend or often disappear completely.
In studies that have evaluated well-formulated very-low-saccharide diets and documented high rates of compliance in individuals with T2D, results accept been nothing brusque of remarkable. Bistrian et al. 32 reported withdrawal of insulin and major weight loss in a matter of weeks in T2D individuals who were fed a very-low-calorie and -sugar diet. Gumbiner et al. 33 fed obese T2D individuals two types of hypocaloric (650 kcal) diets for iii weeks, they were matched for protein but one was much lower in carbohydrate content (24 vs 94 thousand/day). As expected, the lower-saccharide diet resulted in significantly greater levels of circulating ketones (∼three mmol/l), which was strongly associated with a lower hepatic glucose output. Interestingly, there was a stiff inverse correlation betwixt circulating ketones and hepatic glucose output, suggesting that college levels of ketones are associated with more favourable effects on glycaemic control in diabetics. More recently, Boden et al. 34 performed an in-patient study in obese T2D individuals who were fed a low-carbohydrate (<20 grand/day) nutrition for 2 weeks. Plasma glucose savage from seven.5 to 6.3 mmol/l, haemoglobin A1c decreased from 7.3 to six.viii% and there were dramatic improvements (75%) in insulin sensitivity.
In a longer written report35 obese T2D individuals were prescribed a well-formulated ketogenic nutrition for 56 weeks, and meaning improvements in both weight loss and metabolic parameters were seen at 12 weeks and continued throughout the 56 weeks as evidenced by improvements in fasting circulating levels of glucose (−51%), total cholesterol (−29%), high-density lipoprotein–cholesterol (63%), low-density lipoprotein–cholesterol (−33%) and triglycerides (−41%). It is of interest to annotation that in a recent study in overweight not/diabetic subjects, it was reported that during ketosis fasting glucose was not affected, but there was an peak in postal service-prandial blood glucose concentration. This information suggests a unlike upshot of ketosis on glucose homeostasis in diabetic and non-diabetic individuals.21 Other studies support the long-term efficacy of ketogenic diets in managing complications of T2D.36, 37 Although pregnant reductions in fatty mass oftentimes results when individuals restrict sugar, the improvements in glycaemic control, haemoglobin A1c and lipid markers, every bit well as reduced apply or withdrawal of insulin and other medications in many cases, occurs before pregnant weight loss occurs. Moreover, in isocaloric experiments individuals with insulin resistance showed dramatically improved markers of metabolic syndrome than diets lower in fat.27 It is interesting in this respect that a recent extremely large epidemiological study reported that diabetes risk is directly correlated, in an apparently causative manner, with sugar intake alone, independently of weight or sedentary lifestyle.38
In summary, individuals with metabolic syndrome, insulin resistance and T2D (all diseases of carbohydrate intolerance) are likely to see symptomatic as well every bit objective improvements in biomarkers of illness chance if they follow a well-formulated very-low-saccharide diet. Glucose control improves not only because there is less glucose coming in, but also because systemic insulin sensitivity improves as well.
Epilepsy
Since 1920, the ketogenic nutrition has been recognized equally an effective tool in the treatment of severe babyhood epilepsy, but following the introduction of anticonvulsant drugs, the interest in ketogenic diet treatment waned until the 1990s, with subsequent enquiry and clinical trials demonstrating its practical usefulness. Various studies have been carried out to understand its mechanism of action in epilepsy, but until now it remains largely uncertain.5 Several hypotheses have been put forwards trying to explain the mechanism of action of ketogenic diets: (1) a direct anticonvulsant effect of KBs; (2) a reduced neuronal excitability induced by KBs;39 (3) an result on the mammalian target of rapamycin pathway.40 In 2008, Hartman et al. 41 demonstrated the efficacy of a ketogenic diet in the 6-Hz seizure test in mice, just also reported that the protection from seizures was non related to the level of ketosis in the blood, indicating that mechanism(s) of activeness other than those directly linked to the claret concentration of KBs should be investigated. Most researchers suggest that the metabolic mechanism(southward) activated by ketogenic diets (see above) may influence neurotransmitter activity in neurons and this is currently a field of active enquiry. Although the mechanisms of action are non articulate, the ketogenic nutrition is at present considered an established part of an integrative approach, along with drug therapy, in the major epilepsy centres worldwide,42 an important benefit being the reduction of drug apply and concomitant reductions in astringent side furnishings ofttimes associated with antiepileptic agents. The effectiveness of ketogenic diets is strongly supported in a recent Cochrane review where all studies showed a thirty–40% reduction in seizures compared with comparative controls, and the review authors reported that in children the effects were 'comparable to modern antiepileptic drugs'. The main drawback with the ketogenic diet was difficult tolerability and high dropout rates—given the extremely positive results and the severe side furnishings mutual with antiepilepsy medication, the evolution of an easier-to-follow ketogenic diet would exist a worthwhile goal.five
In decision, the role of ketogenic diets in epilepsy treatment is well established and we are confident that this is also the case for weight loss, cardiovascular disease and T2D. The recent research reviewed here demonstrate improvements in many risk factors, such as weight, saturated fats, inflammation and other biomarkers, equally a upshot of consuming well-formulated low-carbohydrate diets, and this work should encourage continued shut examination of their therapeutic value (Figure one).
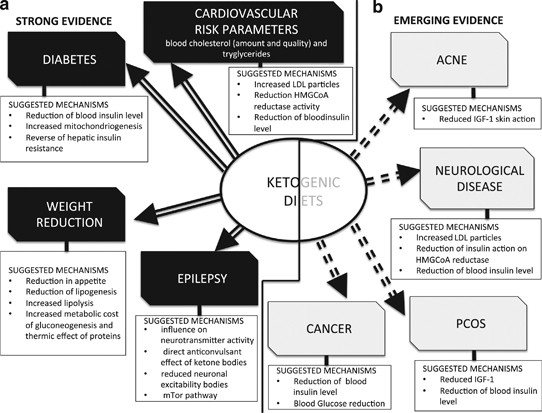
Suggested mechanisms for the therapeutic action of ketogenic diets in pathologies for which there exists potent (a) and emerging (b) evidence.
Emerging testify
Acne
In contempo years there have been an increasing number of studies published, suggesting that at least for certain nutrient types there is a nutritional influence on the development of acne. The negative effects seem to lie in the capacity of some foods/nutrients to stimulate proliferative pathways that in plow stimulate development of acne—doubtable foods include those with a high glycaemic load and milk.11, 43, 44 Other evidence comes from several studies reporting that the prevalence of acne varies significantly betwixt unlike populations and is essentially lower in non-Westernized populations that follow traditional diets,45 a mutual factor among these traditional diets existence a low glycaemic load.46 Various studies accept provided evidence that high-glycaemic-load diets are implicated in the aetiology of acne through their chapters to stimulate insulin, androgen bioavailability and insulin-like growth factor-1 (IGF-1) activeness, whereas the beneficial effects of low-glycaemic-load diets, apart from weight and blood glucose levels, too include improved skin quality.44 The clinical and experimental evidence does in fact suggest means in which insulin can increase androgen product and affect via induction of steroidogenic enzymes,47 the secretion by the pituitary gland of gonadotropin-releasing hormone and the product of sex hormone-binding globulin.48 Insulin is also able to reduce serum levels of IGF-binding protein-1 increasing the effect of IGF-1.49 These insulin-mediated actions can therefore influence diverse factors that underlie the development of acne such as:
- a)
The increased proliferation of basal keratinocytes within the pilosebaceous ducts.
- b)
An abnormal desquamation of the follicular epithelium.
- c)
Increased androgen-mediated sebum production.
- d)
Colonization of the stratum corneum past Propionibacterium acnes and consequent inflammation.46
In summary, there is persuasive, although not yet conclusive, clinical and physiological evidence that the ketogenic diet could be effective in reducing the severity and progression of acne and randomized clinical trials will be required to resolve the upshot.xi
Cancer
Carcinogenesis is a complex process involving multiple sequential mutations, which occur randomly in the DNA of normal cells over many years, even decades, until finally specific genes are mutated and cell growth becomes out of command resulting in the full neoplastic phenotype and oftentimes metastasis. There is evidence that hyperinsulinaemia, hyperglycaemia and chronic inflammation may touch on the neoplastic process through various pathways, including the insulin/IGF-1 pathway, and most cancer cells express insulin and IGF-1 receptors. Insulin has been shown to stimulate mitogenesis (even in cells lacking IGF-1 receptors)fifty and information technology may also contribute past stimulating multiple cancer mechanisms, including proliferation, protection from apoptotic stimuli, invasion and metastasis.51 The IFG1/insulin pathway may besides raise the promotion and progression of many types of cancer cells and facilitate cancer improvidence through angiogenesis.52 Insulin may act directly, but likewise indirectly through IGF-ane, as information technology reduces hepatic IGF-bounden poly peptide-1 and -2 production,53 thereby increasing the levels of circulating, free agile IGF-1, which may have a role in cancerogenesis due to its mitogenic and antiapoptotic activeness.53 Considering the obvious relationship between carbohydrates and insulin (and IGF-1) a connection between carbohydrate and cancer is a possible issue, and some links have been recognized since the 1920s when the Russo-German physician Dr A Braunstein observed that glycosuria falls off notably in diabetic patients who adult cancer.54 Later Warburg et al. 55 of the Kaiser Wilheim Institute fur biologie described what was later on known equally the Warburg effect—where energy is predominantly generated by a high rate of glycolysis followed by lactic acid fermentation in the cytosol, even in the presence of plentiful oxygen.51, 55 The Warburg consequence has been confirmed in many studies and today is a well-established hallmark of many types of cancers, and quickly growing tumour cells typically have glycolytic rates upwards to 200 times higher than those of their normal tissues of origin.56 As stated above, the stimulus of the insulin/IGF-1 pathway is involved in cancer development, but also mitochondrial harm or dysfunction may accept a office.18, 57, 58 Dysfunctional mitochondria may upregulate some oncogenes of the phosphatidylinositol 3-kinase/Akt mammalian target of rapamycin signalling pathway.58 Akt, a downstream of insulin signalling,59 is involved in changes in tumour cell metabolism and increases resistance to apoptosis; it too decreases β-oxidation and increases lipid synthesis in the cytosol.lx Hence, it seems a reasonable possibility that a very-low-saccharide diet could help to reduce the progression of some types of cancer, although at present the evidence is preliminary.61 In the 1980s, seminal animate being studies by Tisdale and colleagues62, 63 demonstrated that a ketogenic diet was capable to reduce tumour size in mice, whereas more recent enquiry has provided testify that ketogenic diets may reduce tumour progression in humans, at least every bit far as gastric and encephalon cancers are concerned.64, 65, 66, 67 Although no randomized controlled trials with VLCKD have yet been conducted on patients and the bulk of evidence in relation to the influence of VLCKD on patient survival is yet anecdotal,68, 69, 70 a very recent paper past Fine et al. 71 suggests that the insulin inhibition acquired by a ketogenic diet could exist a feasible adjunctive treatment for patients with cancer. In summary, perhaps through glucose 'starvation' of tumour cells and past reducing the effect of directly insulin-related actions on cell growth, ketogenic diets show promise as an help in at least some kind of cancer therapy and is deserving of further and deeper investigation—certainly the evidence justifies setting up clinical trials.
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in females, with a high prevalence (half dozen–ten%);72 symptoms include hyperandrogenism, ovulatory dysfunction, obesity, insulin resistance and subfertility. Insulin resistance and related hyperinsulinaemia is actually a very common characteristic affecting well-nigh 65–70% of women with PCOS;73 it is seen most frequently in obese patients, affecting 70–80%, compared with only 20–25% of lean PCOS sufferers.72 Despite this ascertainment, insulin resistance and hyperinsulinaemia appear to be linked to PCOS independently of obesity, and modifications in the normal cellular mechanisms of insulin signalling accept been demonstrated in both lean and obese patients. Furthermore, high blood levels of insulin can deed past increasing androgenous hormonal stimulation of the ovarian theca cells as well as potentiating gonadotropin-stimulated ovarian androgen steroidogenesis—although recent data has suggested that the insulin-induced increase in ovarian hormone secretion is not accompanied by increased steroid metabolism.74 Hyperinsulinaemia may as well bear upon the central actions of androgen by impairing progesterone inhibition of the gonadotropin-releasing hormone pulse generator.75 Insulin has likewise been shown to increase expression of adrenal steroidogenic enzyme mRNA47 equally well as adrenal responsiveness to adrenocorticotropic hormone.76
Women with PCOS frequently demonstrate many of the signs related to metabolic syndrome, such equally insulin resistance, obesity, glucose intolerance, T2D, dyslipidemia and besides loftier levels of inflammation. Suggested treatments include those that reduce insulin resistance/hyperinsulinaemia, such as lifestyle modifications (exercise, diet and weight loss) and pharmacological treatments that include the administration of thiazolidinediones or metformin. It is evident that any interventions that meliorate insulinaemia and reduce body weight may also be effective in reducing hyperandrogenism, normalizing ovulation and reducing the various symptoms of PCOS.
Finally, although we only have preliminary evidence of the positive effects of VLCKD in PCOS,77 there are articulate mechanisms that are consistent with the physiological plausibility of such dietary therapy.
Neurological diseases
Emerging data advise a possible therapeutic utilization of ketogenic diets in multiple neurological disorders apart from epilepsy,78 including caput anguish, neurotrauma, Alzheimer'southward and Parkinson's affliction, sleep disorders, brain cancer, autism and multiple sclerosis.79 Although these diverse diseases are clearly different from each other, a common basis potentially explaining ketogenic diet efficacy could exist a neuroprotective upshot in any disease in which the pathogenesis includes abnormalities in cellular energy utilization, which is a mutual characteristic in many neurological disorders.79 The exact machinery(s) by which a ketogenic diet may human action is however poorly understood; withal, some published reports can provide useful suggestions. For case, KBs were recently reported to act as neuroprotective agents by raising ATP levels and reducing the product of reactive oxygen species in neurological tissues,fourscore together with increased mitochondrial biogenesis, which may help to enhance the regulation of synaptic function.80 Moreover, the increased synthesis of polyunsaturated fatty acids stimulated by a KD may accept a function in the regulation of neuronal membrane excitability: it has been demonstrated, for case, that polyunsaturated fatty acids modulate the excitability of neurons by blocking voltage-gated sodium channels.81 Another possibility is that by reducing glucose metabolism, ketogenic diets may activate anticonvulsant mechanisms, equally has been reported in a rat model.82 In add-on, caloric brake per se has been suggested to exert neuroprotective effects, including improved mitochondrial function, decreased oxidative stress and apoptosis, and inhibition of proinflammatory mediators, such as the cytokines neoplasm necrosis factor-α and interleukins.83 Although promising data have been nerveless (see below), at the present time the existent clinical benefits of ketogenic diets in most neurological diseases remain largely speculative and uncertain, with the significant exception of its utilize in the handling of convulsion diseases.
Alzheimer'southward disease
Patients affected with Alzheimer'due south affliction show a higher incidence of seizures compared with unaffected people,84 and it has recently been reported that neuronal excitability is enhanced,85, 86 and neuronal circuits and mitochondrial homeostasis are altered.87
On the basis of the reports described to a higher place, these results signal a possible role of the ketogenic diet in the handling of Alzheimer'due south disease in the clinic. Supporting evidence is provided past a written report, which reported that at least in selected atmospheric condition a meaning clinical improvement was observed in Alzheimer's patients fed a ketogenic nutrition.88 It was suggested that this was, at least in part, related to improved mitochondrial part secondary to the reported protective effects of KBs confronting the toxic consequences of the exposure of cultured neurons to β-amyloid.89 In an creature model of Alzheimer's disease, transgenic mice consuming a ketogenic diet exhibited amend mitochondrial function and less oxidative stress and β-amyloid deposition when compared with unremarkably fed controls.90 These promising results should encourage further research that is necessary to improve our understanding about the potential benefits of ketogenic diets in this debilitating and, thus far, irreversible affliction.
Parkinson's affliction
The possible beneficial effects of ketogenic diets on mitochondrial activity described to a higher place has also been proposed to explain the improved scores on a standard gravity scale of Parkinson' disease exhibited by some patients.91 In addition, the typical mitochondrial respiratory chain impairment that occurs in beast models of Parkinson'due south disease was reduced past a ketogenic diet;89 however, the real utility of this diet remains largely speculative and uncertain.
Brain trauma
Traumatic brain injury may lead over time to epilepsy. Because of the effective utilise of the ketogenic diet in reducing seizures (see in a higher place), it has been suggested that it may also improve the clinical status in encephalon injury, especially by reducing the incidence of long-term consequences, such as epilepsy.79 Positive furnishings of a ketogenic nutrition have also been reported in reducing the cortical contusion volume in an age-dependent manner in an fauna model of cortical injury, which is related to the maturation-dependent variability in brain ketone metabolism.92 These findings were also supported past the demonstration that a ketogenic diet reduced post-traumatic cognitive and motor part impairment, at least in a rat model.93
The antiepileptogenic activity of the ketogenic nutrition after traumatic encephalon damage is controversial though,94 and further studies are needed to increment related noesis.
Amyotrophic lateral sclerosis
Dysfunction in energy production, that is, mitochondrial role harm, is likely to have a role in the pathogenesis of many neurodegenerative diseases, maybe including amyotrophic lateral sclerosis. On this basis, a ketogenic diet has been proposed as a collateral therapeutic arroyo in this disease.95 Studies by Zhao et al. 96 revealed both histological and functional improvements in an brute model of amyotrophic lateral sclerosis when a ketogenic nutrition was given compared with when given a control nutrition. Although survival time was not increased, a college motor neuron count and lower motor role impairment was reported among the findings.
Even so, directly experimentation and clinical trials in humans are still lacking at the nowadays time, and to avoid the possibility of generating false hopes the preliminary data from animal models obviously have to be considered very charily.
The event of a ketogenic diet on respiratory function
The metabolic effects of a ketogenic diet imply a higher-than-usual oxidation of fats, which leads in plow to reduced respiratory commutation ratio values.20, 97 Metabolic carbon dioxide output may be calculated as the product of alveolar ventilation multiplied by the fractional alveolar carbon dioxide concentration. Pulmonary ventilation differs from alveolar ventilation merely by the amount of physiological dead infinite, and at that place is no reason to suspect a change in physiological dead infinite when a dietary manipulation is practical. Hence, post-obit a ketogenic diet-induced decrement of the respiratory exchange ratio and of metabolic carbon dioxide output, a decrease in arterial carbon dioxide partial pressure or of pulmonary ventilation, or of both, is expected. If verified, these effects might exist useful in the handling of patients with respiratory failure; however, this aspect of the ketogenic diet remains to be investigated. Sabapathy et al. 98 observed that the reduction in muscle glycogen content acquired a respiratory commutation ratio decrement, which was associated with reduced carbon dioxide partial pressure and constancy of pulmonary ventilation. These findings at least suggest potential useful effects of this diet in patients with increased carbon dioxide, arterial partial-force per unit area values as a consequence of respiratory failure. Of course, more studies are needed to verify this working hypothesis.
Potential risks of ketogenic diets
If nosotros equate de facto ketogenic diets with high-poly peptide diets (which is non ever right) then the risks proposed by critics of this type of dietary arroyo are essentially those of possible kidney damage due to high levels of nitrogen excretion during poly peptide metabolism, which tin crusade an increase in glomerular force per unit area and hyperfiltration.12 At that place is non wide understanding betwixt studies; however, some infer the possibility of renal damage from animal studies,99, 100 whereas others, looking at both animate being models, meta-analyses and human studies, suggest that even high levels of protein in the diet do non impairment renal function.101, 102 In subjects with intact renal part, higher dietary poly peptide levels acquired some functional and morphological adaptations without negative effects.103 There may actually be renal-related effects, but on blood force per unit area rather than morphological damage. The amino acids involved in gluconeogenesis and/or production of urea in full general have blood-pressure-lowering effects, whereas acidifying amino acids tend to cause a rise in blood pressure. Subjects with renal insufficiency, even subclinical, kidney transplant patients and people with metabolic syndrome or other obesity-related atmospheric condition, will be more susceptible to the hypertensive effect of amino acids, especially of the sulphated variety.104 The well-documented correlation between obesity and reduced nephron quantity on raised blood pressure puts subjects with T2D or metabolic syndrome at risk, even if in diabetics with kidney damage the effects are not always consequent with the hypothesis.12,105,106 In fact, although some authors have reported a positive influence of a reduction in protein intake from 1.2 to 0.ix m/kg, over the short term, on albuminuria in T2D,107 the aforementioned authors have subsequently stated instead that dietary poly peptide restriction is neither necessary nor useful over the long term.108
Moreover, information technology should be noted that ketogenic diets are only relatively high in protein18, 106 and that some recent studies have demonstrated that VLCKD can even crusade a regression of diabetic nephropathy in mice.109 With regard to possible acidosis during VLCKD, as the concentration of KBs never rises above 8 mmol/50ten this risk is well-nigh inexistent in subjects with normal insulin function.
Conclusions
Ketogenic diets are commonly considered to be a useful tool for weight control and many studies suggest that they could exist more than efficient than low-fat diets, although there is non cyclopedia in the literature about their accented effectiveness and even some doubts raised well-nigh safety. But there is a 'hidden face up' of the ketogenic diet: its broader therapeutic action. There are new and heady scenarios about the use of ketogenic diets, equally discussed in this review, in cancer, T2D, PCOS, cardiovascular and neurological diseases. Further studies are warranted to investigate more in detail the potential therapeutic mechanisms, its effectiveness and safety, and we would invite all researchers to face up this claiming without prejudice.
References
-
Atkins RC . Dr Atkins' Diet Revolution: The High Calorie Way to Stay Sparse Forever. D. McKay Co: New York, NY, U.s., 1972.
-
WHO. Medicines: Corruption and Pharmaceuticals. WHO Fact Sheet, WHO, 2009.
-
Veech RL . The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 2004; seventy: 309–319.
-
Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr . Brain metabolism during fasting. J Clin Invest 1967; 46: 1589–1595.
-
Kessler SK, Neal EG, Camfield CS, Kossoff EH . Dietary therapies for epilepsy: future research. Epilepsy Behav 2011; 22: 17–22.
-
Fukao T, Lopaschuk GD, Mitchell GA . Pathways and command of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 243–251.
-
Veldhorst MA, Westerterp-Plantenga MS, Westerterp KR . Gluconeogenesis and energy expenditure later on a loftier-protein, carbohydrate-free diet. Am J Clin Nutr 2009; 90: 519–526.
-
Krebs HA . The regulation of the release of ketone bodies past the liver. Adv Enzyme Regul 1966; four: 339–354.
-
Paoli A, Canato M, Toniolo Fifty, Bargossi AM, Neri M, Mediati M et al. The ketogenic nutrition: an underappreciated therapeutic option? Clin Ter 2011; 162: e145–e153.
-
Cahill GF Jr . Fuel metabolism in starvation. Annu Rev Nutr 2006; 26: one–22.
-
Paoli A, Grimaldi Chiliad, Toniolo Fifty, Canato 1000, Bianco A, Fratter A . Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol 2012; 25: 111–117.
-
Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR . Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr 2009; 29: 21–41.
-
Feinman RD, Fine EJ . Nonequilibrium thermodynamics and energy efficiency in weight loss diets. Theor Biol Med Model 2007; 4: 27.
-
Freedman MR, King J, Kennedy E . Popular diets: A scientific review. Obes Res 2001; ix (Suppl one), 1S–40S.
-
Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA . A randomized trial comparison a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular chance factors in healthy women. J Clin Endocrinol Metab 2003; 88: 1617–1623.
-
Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR et al. Comparison of the atkins, zone, ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A TO Z weight loss study: a randomized trial. JAMA 2007; 297: 969–977.
-
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow South, Greenberg I et al. Weight loss with a low-carbohydrate, mediterranean, or low-fat diet. N Engl J Med 2008; 359: 229–241.
-
Fine EJ, Feinman RD . Thermodynamics of weight loss diets. Nutr Metab (Lond) 2004; one: xv.
-
Halton TL, Hu FB . The furnishings of high poly peptide diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 2004; 23: 373–385.
-
Paoli A, Grimaldi K, Bianco A, Lodi A, Cenci Fifty, Parmagnani A . Medium term effects of a ketogenic diet and a mediterranean diet on resting energy expenditure and respiratory ratio. BMC Proceedings 2012; 6, (Suppl 3): P37.
-
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A et al. Ketosis and appetite-mediating nutrients and hormones subsequently weight loss. Eur J Clin Nutr 2013;, e-pub ahead of print 1 May 2013; doi:x.1038/ejcn.2013.xc.
-
Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K et al. Poly peptide-induced satiety: furnishings and mechanisms of different proteins. Physiol Behav 2008; 94: 300–307.
-
Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE . Effects of a high-protein ketogenic diet on hunger, ambition, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87: 44–55.
-
Paoli A, Cenci L, Fancelli M, Parmagnani A, Fratter A, Cucchi A et al. Ketogenic diet and phytoextracts comparison of the efficacy of mediterranean, zone and tisanoreica diet on some health risk factors. Agro Food Ind Hullo-Tech 2010; 21: 24.
-
Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ et al. Effects of low-sugar vs low-fat diets on weight loss and cardiovascular adventure factors: a meta-analysis of randomized controlled trials. Arch Intern Med 2006; 166: 285–293.
-
Blackburn GL, Phillips JC, Morreale S . Physician's guide to popular low-sugar weight-loss diets. Cleve Clin J Med 2001; 68: 761–766. 768–ix, 773–iv.
-
Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ et al. Carbohydrate restriction has a more favorable touch on on the metabolic syndrome than a low fatty nutrition. Lipids 2009; 44: 297–309.
-
Sharman MJ, Kraemer WJ, Love DM, Avery NG, Gomez AL, Scheett TP et al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr 2002; 132: 1879–1885.
-
Volek JS, Sharman MJ, Forsythe CE . Modification of lipoproteins by very low-saccharide diets. J Nutr 2005; 135: 1339–1342.
-
Jornayvaz FR, Samuel VT, Shulman GI . The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver affliction associated with the metabolic syndrome. Annu Rev Nutr 2010; 30: 273–290.
-
Hellerstein MK . De novo lipogenesis in humans: Metabolic and regulatory aspects. Eur J Clin Nutr 1999; 53 (Suppl ane), S53–S65.
-
Bistrian BR, Blackburn GL, Flatt JP, Sizer J, Scrimshaw NS, Sherman M . Nitrogen metabolism and insulin requirements in obese diabetic adults on a protein-sparing modified fast. Diabetes 1976; 25: 494–504.
-
Gumbiner B, Wendel JA, McDermott MP . Effects of diet limerick and ketosis on glycemia during very-low-energy-diet therapy in obese patients with not-insulin-dependent diabetes mellitus. Am J Clin Nutr 1996; 63: 110–115.
-
Boden Thousand, Sargrad K, Homko C, Mozzoli M, Stein TP . Result of a low-carbohydrate nutrition on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005; 142: 403–411.
-
Dashti HM, Al-Zaid NS, Mathew TC, Al-Mousawi M, Talib H, Asfar SK et al. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol Prison cell Biochem 2006; 286: 1–nine.
-
Yancy WS Jr, Foy Yard, Chalecki AM, Vernon MC, Westman EC . A low-saccharide, ketogenic nutrition to treat blazon 2 diabetes. Nutr Metab (Lond) 2005; ii: 34.
-
Nielsen JV, Joensson EA . Depression-saccharide diet in type 2 diabetes: Stable improvement of bodyweight and glycemic control during 44 months follow-up. Nutr Metab (Lond) 2008; 5: 14.
-
Basu South, Yoffe P, Hills Northward, Lustig RH . The relationship of sugar to population-level diabetes prevalence: An econometric assay of repeated cross-sectional data. PLoS I 2013; eight: e57873.
-
Hartman AL, Gasior M, Vining EP, Rogawski MA . The neuropharmacology of the ketogenic diet. Pediatr Neurol 2007; 36: 281–292.
-
McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong Thousand . The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011; 52: e7–e11.
-
Hartman AL, Lyle M, Rogawski MA, Gasior M . Efficacy of the ketogenic diet in the half dozen-hz seizure test. Epilepsia 2008; 49: 334–339.
-
Kossoff E . The fat is in the fire: ketogenic diet for refractory status epilepticus. Epilepsy Curr 2011; 11: 88–89.
-
Cordain L, Eaton SB, Sebastian A, Isle of mann N, Lindeberg South, Watkins BA et al. Origins and evolution of the western diet: wellness implications for the 21st century. Am J Clin Nutr 2005; 81: 341–354.
-
Smith RN, Isle of man NJ, Braue A, Makelainen H, Varigos GA . The upshot of a high-poly peptide, depression glycemic-load diet versus a conventional, high glycemic-load nutrition on biochemical parameters associated with acne vulgaris: A randomized, investigator-masked, controlled trial. J Am Acad Dermatol 2007; 57: 247–256.
-
Smith R, Isle of mann N . Acne in adolescence: a role for nutrition? Nutr Diet 2007; 64: S147–S149.
-
Cordain Fifty . Implications for the role of nutrition in acne. Semin Cutan Med Surg 2005; 24: 84–91.
-
Kristiansen SB, Endoh A, Casson PR, Buster JE, Hornsby PJ . Induction of steroidogenic enzyme genes by insulin and IGF-I in cultured adult homo adrenocortical cells. Steroids 1997; 62: 258–265.
-
Goodman-Gruen D, Barrett-Connor Due east . Sexual practice hormone-binding globulin and glucose tolerance in postmenopausal women. the rancho bernardo report. Diabetes Care 1997; 20: 645–649.
-
Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD . Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-one. J Biol Chem 1991; 266: 18868–18876.
-
Denley A, Carroll JM, Brierley GV, Cosgrove L, Wallace J, Forbes B et al. Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol Prison cell Biol 2007; 27: 3569–3577.
-
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA et al. Diabetes and cancer: a consensus report. CA Cancer J Clin 2010; 60: 207–221.
-
Rose DP, Vona-Davis L . The cellular and molecular mechanisms by which insulin influences breast cancer take a chance and progression. Endocr Relat Cancer 2012; nineteen: R225–R241.
-
Renehan AG, Frystyk J, Flyvbjerg A . Obesity and cancer gamble: the role of the insulin-IGF axis. Trends Endocrinol Metab 2006; 17: 328–336.
-
AA.VV. Inquiry in cancer. Scientific discipline 1925; 62, x+xii+xiv.
-
Warburg O, Wind F, Negelein East . The metabolism of tumors in the body. J Gen Physiol 1927; 8: 519–530.
-
Hanahan D, Weinberg RA . Hallmarks of cancer: the side by side generation. Cell 2011; 144: 646–674.
-
Warburg O . On respiratory impairment in cancer cells. Science 1956; 124: 269–270.
-
Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS et al. Mitochondrial respiration defects in cancer cells cause activation of akt survival pathway through a redox-mediated machinery. J Cell Biol 2006; 175: 913–923.
-
Sandri 1000, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan M et al. Signalling pathways regulating muscle mass in ageing skeletal muscle. the function of the IGF1-akt-mTOR-FoxO pathway. Biogerontology 2013;, e-pub ahead of print 19 May 2013.
-
Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM . Expression of constitutively activated akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res 2003; 44: 1100–1112.
-
Klement RJ, Kammerer U . Is at that place a part for carbohydrate brake in the treatment and prevention of cancer? Nutr Metab (Lond) 2011; viii: 75.
-
Tisdale MJ, Brennan RA, Fearon KC . Reduction of weight loss and tumour size in a cachexia model by a high fatty nutrition. Br J Cancer 1987; 56: 39–43.
-
Beck SA, Tisdale MJ . Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer 1989; 59: 677–681.
-
Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY et al. A low sugar, loftier poly peptide nutrition slows tumor growth and prevents cancer initiation. Cancer Res 2011; 71: 4484–4493.
-
Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R et al. Growth of homo gastric cancer cells in nude mice is delayed past a ketogenic diet supplemented with omega-3 fatty acids and medium-concatenation triglycerides. BMC Cancer 2008; 8: 122.
-
Seyfried BT, Kiebish M, Marsh J, Mukherjee P . Targeting free energy metabolism in encephalon cancer through calorie restriction and the ketogenic diet. J Cancer Res Ther 2009; 5 (Suppl 1), S7–S15.
-
Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN . The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007; four: five.
-
Schmidt M, Pfetzer N, Schwab K, Strauss I, Kammerer U . Furnishings of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond) 2011; viii: 54.
-
Nebeling LC, Lerner E . Implementing a ketogenic nutrition based on medium-chain triglyceride oil in pediatric patients with cancer. J Am Diet Assoc 1995; 95: 693–697.
-
Nebeling LC, Miraldi F, Shurin SB, Lerner E . Furnishings of a ketogenic nutrition on tumor metabolism and nutritional status in pediatric oncology patients: Two case reports. J Am Coll Nutr 1995; xiv: 202–208.
-
Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf Southward, Romano MC, Tomuta N et al. Targeting insulin inhibition as a metabolic therapy in avant-garde cancer: a pilot safety and feasibility dietary trial in x patients. Nutrition 2012; 28: 1028–1035.
-
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R et al. Consensus on women'southward health aspects of polycystic ovary syndrome (PCOS): the amsterdam ESHRE/ASRM-sponsored tertiary PCOS consensus workshop group. Fertil Steril 2012; 97: 28–38. . e25.
-
DeUgarte CM, Bartolucci AA, Azziz R . Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005; 83: 1454–1460.
-
Tosi F, Negri C, Perrone F, Dorizzi R, Castello R, Bonora Due east et al. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2012; 97: 1712–1719.
-
Bare SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ et al. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls--implications for regulation of pubertal maturation. J Clin Endocrinol Metab 2009; 94: 2360–2366.
-
Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun Eastward et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: credible relative impairment of 17,twenty-lyase activity. J Clin Endocrinol Metab 1996; 81: 881–886.
-
Mavropoulos JC, Yancy WS, Hepburn J, Westman EC . The furnishings of a depression-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond) 2005; 2: 35.
-
Baranano KW, Hartman AL . The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol 2008; ten: 410–419.
-
Stafstrom CE, Rho JM . The ketogenic nutrition as a treatment epitome for diverse neurological disorders. Front Pharmacol 2012; 3: 59.
-
Bough KJ, Rho JM . Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 2007; 48: 43–58.
-
Huffman J, Kossoff EH . State of the ketogenic nutrition(s) in epilepsy. Curr Neurol Neurosci Rep 2006; six: 332–340.
-
Garriga-Canut Thou, Schoenike B, Qazi R, Bergendahl 1000, Daley TJ, Pfender RM et al. 2-deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci 2006; nine: 1382–1387.
-
Maalouf M, Rho JM, Mattson MP . The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev 2009; 59: 293–315.
-
Palop JJ, Mucke L . Epilepsy and cognitive impairments in alzheimer affliction. Curvation Neurol 2009; 66: 435–440.
-
Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F et al. Amyloid-beta/fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of alzheimer'south affliction. J Neurosci 2011; 31: 700–711.
-
Noebels J . A perfect storm: converging paths of epilepsy and alzheimer's dementia intersect in the hippocampal formation. Epilepsia 2011; 52 (Suppl one), 39–46.
-
Kapogiannis D, Mattson MP . Disrupted energy metabolism and neuronal circuit dysfunction in cognitive harm and alzheimer's disease. Lancet Neurol 2011; 10: 187–198.
-
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC . Study of the ketogenic agent Ac-1202 in mild to moderate alzheimer'due south disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009; 6: 31.
-
Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL . D-beta-hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci Us 2000; 97: 5440–5444.
-
Van der Auwera I, Wera S, Van Leuven F, Henderson ST . A ketogenic diet reduces amyloid beta twoscore and 42 in a mouse model of alzheimer's affliction. Nutr Metab (Lond) 2005; ii: 28.
-
Vanitallie TB, Nonas C, Di Rocco A, Boyar M, Hyams Thousand, Heymsfield SB . Treatment of parkinson illness with nutrition-induced hyperketonemia: a feasibility study. Neurology 2005; 64: 728–730.
-
Prins ML, Fujima LS, Hovda DA . Age-dependent reduction of cortical contusion volume by ketones afterwards traumatic encephalon injury. J Neurosci Res 2005; 82: 413–420.
-
Appelberg KS, Hovda DA, Prins ML . The effects of a ketogenic diet on behavioral outcome subsequently controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma 2009; 26: 497–506.
-
Schwartzkroin PA, Wenzel HJ, Lyeth BG, Poon CC, Delance A, Van KC et al. Does ketogenic diet alter seizure sensitivity and jail cell loss following fluid percussion injury? Epilepsy Res 2010; 92: 74–84.
-
Siva N . Can ketogenic diet slow progression of ALS? Lancet Neurol 2006; v: 476.
-
Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho Fifty, Suh J et al. A ketogenic nutrition every bit a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci 2006; vii: 29.
-
Tagliabue A, Bertoli S, Trentani C, Borrelli P, Veggiotti P . Effects of the ketogenic nutrition on nutritional condition, resting energy expenditure, and substrate oxidation in patients with medically refractory epilepsy: A half dozen-month prospective observational study. Clin Nutr 2012; 31: 246–249.
-
Sabapathy S, Morris NR, Schneider DA . Ventilatory and gas-exchange responses to incremental practice performed with reduced muscle glycogen content. J Sci Med Sport 2006; 9: 267–273.
-
Jia Y, Hwang SY, House JD, Ogborn MR, Weiler HA, O M et al. Long-term high intake of whole proteins results in renal damage in pigs. J Nutr 2010; 140: 1646–1652.
-
Wakefield AP, Firm JD, Ogborn MR, Weiler HA, Aukema HM . A diet with 35% of energy from poly peptide leads to kidney harm in female sprague-dawley rats. Br J Nutr 2011; one–8.
-
Skov AR, Haulrik N, Toubro S, Molgaard C, Astrup A . Result of protein intake on bone mineralization during weight loss: A 6-month trial. Obes Res 2002; 10: 432–438.
-
Martin WF, Armstrong LE, Rodriguez NR . Dietary protein intake and renal part. Nutr Metab (Lond) 2005; 2: 25.
-
Welle S, Nair KS . Relationship of resting metabolic rate to body composition and protein turnover. Am J Physiol 1990; 258: E990–E998.
-
Praga Chiliad . Synergy of low nephron number and obesity: A new focus on hyperfiltration nephropathy. Nephrol Dial Transplant 2005; 20: 2594–2597.
-
Eisenstein J, Roberts SB, Dallal M, Saltzman East . Loftier-protein weight-loss diets: are they rubber and do they work? A review of the experimental and epidemiologic data. Nutr Rev 2002; 60: 189–200.
-
Westerterp-Plantenga MS . How are normal, high- or low-protein diets defined? Br J Nutr 2007; 97: 217–218.
-
Pijls LT, de Vries H, Donker AJ, van Eijk JT . The outcome of protein brake on albuminuria in patients with type 2 diabetes mellitus: A randomized trial. Nephrol Dial Transplant 1999; 14: 1445–1453.
-
Pijls LT, de Vries H, van Eijk JT, Donker AJ . Protein restriction, glomerular filtration rate and albuminuria in patients with type 2 diabetes mellitus: a randomized trial. Eur J Clin Nutr 2002; 56: 1200–1207.
-
Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV . Reversal of diabetic nephropathy past a ketogenic diet. PLoS One 2011; six: e18604.
Author data
Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of involvement.
Rights and permissions
This piece of work is licensed nether a Artistic Eatables Attribution iii.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
Reprints and Permissions
About this article
Cite this article
Paoli, A., Rubini, A., Volek, J. et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 67, 789–796 (2013). https://doi.org/x.1038/ejcn.2013.116
-
Received:
-
Revised:
-
Accustomed:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/ejcn.2013.116
Keywords
- ketogenic diet
- cancer
- diabetes
- neurological diseases
- obesity
- cardiovascular diseases
Further reading
Source: https://www.nature.com/articles/ejcn2013116
0 Response to "Beyond Weight Loss a Review of the Therapeutic Uses of Verylow Carbohydrate Ketogenic Diets"
Post a Comment